Chemistry - Molar Mass
$0.99
1.3for iPhone, iPad and more
9.9
1 Ratings
Thundercloud Consulting, LLC
Developer
14.9 MB
Size
Mar 16, 2021
Update Date
Education
Category
4+
Age Rating
Age Rating
Chemistry - Molar Mass Screenshots
About Chemistry - Molar Mass
In chemistry the numbers of atoms, molecules, or formula units, amount of substance, in a sample is reported in the SI unit “mole” (abbreviated mol). One mole contains exactly 6.02214076 x 10^23 elementary entities.This number is known as Avogadro's constant.
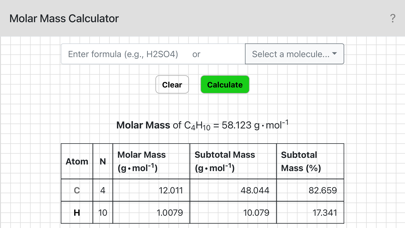
A chemical substance may consist of atoms of a single element (e.g., O2), or of different elements (e.g., CO2). The molar mass of an element is the mass per mole of its atoms. The molar mass of a compound is the mass per mole of its molecules. The molar mass of an ionic compound is mass per mole of its formula units. To calculate the molar mass of a substance we add up the individual molar masses of the constituent atoms.
Note: In nature a sample of an element contains isotopes. Isotopes are atoms with the same number of protons in the nucleus (atomic number), but different masses. The difference in mass between isotopes is the result of different numbers of neutrons. The value of the molar mass of an element used in chemical calculations takes the natural abundance of isotopes into account (i.e., the molar mass is the weighted sum of the isotopic masses found in a typical sample).
To use this calculator enter a chemical formula and tap OK, or alternatively, select a sample molecule from the menu. For example, if you needed the molar mass of sulfuric acid you would enter H2SO4. Parentheses can also be used: the formula for pentane could be entered as CH3(CH2)3CH3 or CH3CH2CH2CH2CH3.
A chemical substance may consist of atoms of a single element (e.g., O2), or of different elements (e.g., CO2). The molar mass of an element is the mass per mole of its atoms. The molar mass of a compound is the mass per mole of its molecules. The molar mass of an ionic compound is mass per mole of its formula units. To calculate the molar mass of a substance we add up the individual molar masses of the constituent atoms.
Note: In nature a sample of an element contains isotopes. Isotopes are atoms with the same number of protons in the nucleus (atomic number), but different masses. The difference in mass between isotopes is the result of different numbers of neutrons. The value of the molar mass of an element used in chemical calculations takes the natural abundance of isotopes into account (i.e., the molar mass is the weighted sum of the isotopic masses found in a typical sample).
To use this calculator enter a chemical formula and tap OK, or alternatively, select a sample molecule from the menu. For example, if you needed the molar mass of sulfuric acid you would enter H2SO4. Parentheses can also be used: the formula for pentane could be entered as CH3(CH2)3CH3 or CH3CH2CH2CH2CH3.
Show More
What's New in the Latest Version 1.3
Last updated on Mar 16, 2021
Old Versions
Revised code and UX.
Show More
Version History
1.3
Mar 16, 2021
Revised code and UX.
1.2.1
Mar 5, 2017
Updated code base and user experience. New icon.
1.1.1
Oct 2, 2015
Added iOS 9 support.
1.1.0
Oct 6, 2014
iPod Touch / iPhone support. Bug fixes.
1.0.2
May 6, 2013
Code updates and interface makeover.
1.0
Mar 7, 2012
Chemistry - Molar Mass FAQ
Click here to learn how to download Chemistry - Molar Mass in restricted country or region.
Check the following list to see the minimum requirements of Chemistry - Molar Mass.
iPhone
Requires iOS 10.2 or later.
iPad
Requires iPadOS 10.2 or later.
iPod touch
Requires iOS 10.2 or later.
Chemistry - Molar Mass supports English