KCENTRA Quick Guide
KCENTRA Quick Guide
Free
1.13for iPhone, iPad and more
Age Rating
KCENTRA Quick Guide Screenshots
About KCENTRA Quick Guide
Please see the Kcentra® app information below.
WARNING: ARTERIAL AND VENOUS THROMBOEMBOLIC COMPLICATIONS
Patients being treated with Vitamin K antagonist therapy have underlying disease states that predispose them to thromboembolic events. Potential benefits of reversing VKA should be weighed against the risk of thromboembolic events, especially in patients with history of such events. Resumption of anticoagulation therapy should be carefully considered once the risk of thromboembolic events outweighs the risk of acute bleeding. Both fatal and nonfatal arterial and venous thromboembolic complications have been reported in clinical trials and postmarketing surveillance. Monitor patients receiving Kcentra, and inform them of signs and symptoms of thromboembolic events. Kcentra was not studied in subjects who had a thromboembolic event, myocardial infarction, disseminated intravascular coagulation, cerebral vascular accident, transient ischemic attack, unstable angina pectoris, or severe peripheral vascular disease within the prior 3 months. Kcentra might not be suitable for patients with thromboembolic events in the prior 3 months.
Kcentra is contraindicated in patients with known anaphylactic or severe systemic reactions to Kcentra or any of its components (including heparin, Factors II, VII, IX, X, Proteins C and S, Antithrombin III and human albumin). Kcentra is also contraindicated in patients with disseminated intravascular coagulation. Because Kcentra contains heparin, it is contraindicated in patients with heparin-induced thrombocytopenia (HIT).
Hypersensitivity reactions to Kcentra may occur. If patient experiences severe allergic or anaphylactic type reactions, discontinue administration and institute appropriate treatment.
In clinical trials, the most frequent (≥2.8%) adverse reactions observed in subjects receiving Kcentra were headache, nausea/vomiting, hypotension, and anemia. The most serious adverse reactions were thromboembolic events, including stroke, pulmonary embolism and deep vein thrombosis.
Kcentra is made from human blood and may carry a risk of transmitting infectious agents, e.g., viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent, and theoretically, the Creutzfeldt-Jakob disease (CJD) agent.
Kcentra®, Prothrombin Complex Concentrate (Human), is a blood coagulation factor replacement product indicated for the urgent reversal of acquired coagulation factor deficiency induced by Vitamin K antagonist (VKA—eg, warfarin) therapy in adult patients with acute major bleeding or the need for urgent surgery or other invasive procedure. Kcentra is for intravenous use only.
------------------------------------
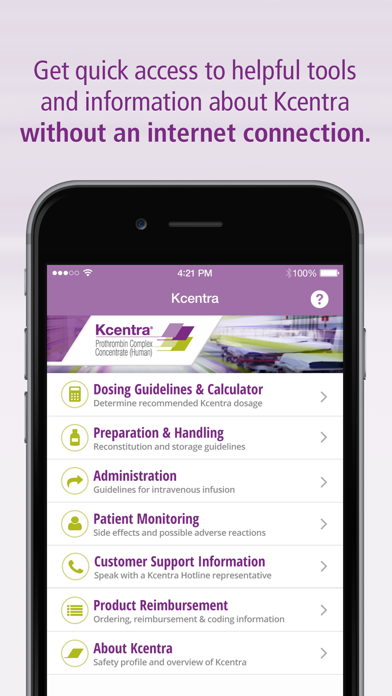
The Kcentra App provides fast access to helpful tools and information from the Kcentra website without the need for an internet connection.
APP FEATURES:
• Dosing calculator and guidelines
• Reconstitution instructional video and step-by-step guide
• Administration guidelines
• Patient monitoring information
• Customer support information
• Product reimbursement information
• Product overview
• Notification center
• Personal bookmarking system
Please see full Prescribing Information and Important Safety Information, including boxed warning at www.Kcentra.com.
WARNING: ARTERIAL AND VENOUS THROMBOEMBOLIC COMPLICATIONS
Patients being treated with Vitamin K antagonist therapy have underlying disease states that predispose them to thromboembolic events. Potential benefits of reversing VKA should be weighed against the risk of thromboembolic events, especially in patients with history of such events. Resumption of anticoagulation therapy should be carefully considered once the risk of thromboembolic events outweighs the risk of acute bleeding. Both fatal and nonfatal arterial and venous thromboembolic complications have been reported in clinical trials and postmarketing surveillance. Monitor patients receiving Kcentra, and inform them of signs and symptoms of thromboembolic events. Kcentra was not studied in subjects who had a thromboembolic event, myocardial infarction, disseminated intravascular coagulation, cerebral vascular accident, transient ischemic attack, unstable angina pectoris, or severe peripheral vascular disease within the prior 3 months. Kcentra might not be suitable for patients with thromboembolic events in the prior 3 months.
Kcentra is contraindicated in patients with known anaphylactic or severe systemic reactions to Kcentra or any of its components (including heparin, Factors II, VII, IX, X, Proteins C and S, Antithrombin III and human albumin). Kcentra is also contraindicated in patients with disseminated intravascular coagulation. Because Kcentra contains heparin, it is contraindicated in patients with heparin-induced thrombocytopenia (HIT).
Hypersensitivity reactions to Kcentra may occur. If patient experiences severe allergic or anaphylactic type reactions, discontinue administration and institute appropriate treatment.
In clinical trials, the most frequent (≥2.8%) adverse reactions observed in subjects receiving Kcentra were headache, nausea/vomiting, hypotension, and anemia. The most serious adverse reactions were thromboembolic events, including stroke, pulmonary embolism and deep vein thrombosis.
Kcentra is made from human blood and may carry a risk of transmitting infectious agents, e.g., viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent, and theoretically, the Creutzfeldt-Jakob disease (CJD) agent.
Kcentra®, Prothrombin Complex Concentrate (Human), is a blood coagulation factor replacement product indicated for the urgent reversal of acquired coagulation factor deficiency induced by Vitamin K antagonist (VKA—eg, warfarin) therapy in adult patients with acute major bleeding or the need for urgent surgery or other invasive procedure. Kcentra is for intravenous use only.
------------------------------------
The Kcentra App provides fast access to helpful tools and information from the Kcentra website without the need for an internet connection.
APP FEATURES:
• Dosing calculator and guidelines
• Reconstitution instructional video and step-by-step guide
• Administration guidelines
• Patient monitoring information
• Customer support information
• Product reimbursement information
• Product overview
• Notification center
• Personal bookmarking system
Please see full Prescribing Information and Important Safety Information, including boxed warning at www.Kcentra.com.
Show More
What's New in the Latest Version 1.13
Last updated on Sep 12, 2023
Old Versions
Minor updates
Show More
Version History
1.13
Sep 12, 2023
Minor updates
1.7
Feb 8, 2023
Minor update in resources.
1.6
Apr 2, 2022
minor update in resources
1.5.2
Mar 19, 2019
minor update in resources
1.5.1
Sep 6, 2018
Minor update
1.5
Aug 30, 2018
Additional materials
1.4
Mar 8, 2018
Updated reconstitution guide
1.3
Nov 21, 2017
Updated Prescribing Information
1.2
Apr 21, 2017
Updated Prescribing Information
1.1
Oct 1, 2016
Updated product reimbursement information
1.0.2
Jan 27, 2016
Updated prescribing information.
1.0
Jun 20, 2015
KCENTRA Quick Guide FAQ
Click here to learn how to download KCENTRA Quick Guide in restricted country or region.
Check the following list to see the minimum requirements of KCENTRA Quick Guide.
iPhone
Requires iOS 11.0 or later.
iPad
Requires iPadOS 11.0 or later.
iPod touch
Requires iOS 11.0 or later.
KCENTRA Quick Guide supports English